 |
High Paying
US Jobs, Economic
Empowerment
&
Revitalization
of
States through Federal Power |
"Critical Field"
Technology Innovation
&
Maximized Profits on
R&D and Investments |
Prevention of Biochemical
and Nuclear Proliferation
&
Homeland Security
Technologies |
Expedited Pain Drug Discovery
Methods and Equipment
by Dr. Michael Nemenov
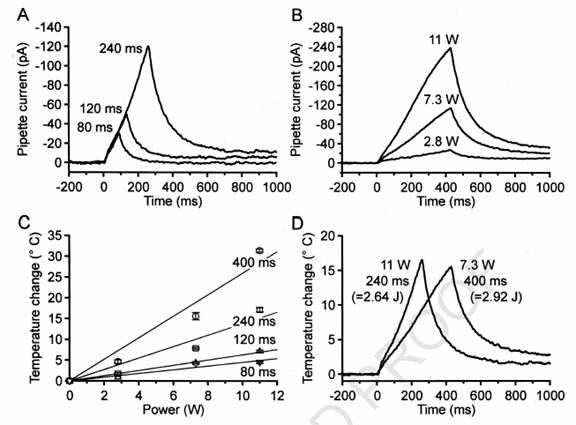
Fig.
1. Diode laser pulses induce ramp-shaped heat stimuli. Time courses of diode
laser induced temperature changes were recorded with open patch pipettes.
Each
trace was averaged from two single measurements, filtered with an eight-pole
Bessel filter (50 Hz) and a notch filter (center frequency, 50 Hz; width, 10
Hz). (A) Peak temperature was always reached at the end of the laser pulse, and
was a function of pulse duration (power: 11 W). (B) Peak temperature was also a
function of laser power (duration: 400 ms). (C) Calibration diagram for one
pipette (mean ^ SEM), based on the average
temperature coefficient of 20.14
^ 0.018C
pA21 determined in a previous
study (Schwarz et al., 2000). For any given pulse duration, peak temperature was
proportional to the applied laser power (W 400
ms; A 240 ms; K 120
ms; L 80 ms). Note that either 11 Wfor 240 ms or 7.3
Wfor 400 ms led to nearly the same peak temperature. (D) By simultaneously
changing power and duration of the laser pulse, the same peak temperature was
achieved with different slopes.
Inward
currents in primary nociceptive neurons of the rat and pain sensations in humans
elicited by infrared diode laser pulses.
Wolfgang
Greffratha, Michael I. Nemenovb,
Stefan Schwarza, Ulf Baumga¨rtnera,
Hagen
Vogela,
Lars
Arendt-Nielsenc, Rolf-Detlef Treedea,*
aInstitute
of Physiology and Pathophysiology,
Johannes
Gutenberg
University
,
Saarstrasse 21, D-55099 Mainz, Germany
bLaser
Medical Center, I.P. Pavlov State Medical University, Lev Tolstoi Street 6/8,
St. Petersburg 197022-1, Russia
cCenter
for Sensory–Motor Interaction, Aalborg University, Fredrik Bajers Vej 7, D3,
DK-9220 Aalborg, Denmark
Radiant
heat is often used to study nociception in vivo. We now used infrared radiation
generated by a diode laser stimulator (wavelength 980 nm) to investigate
transduction mechanisms for noxious heat stimuli in acutely dissociated dorsal
root ganglion (DRG) neurons of rats in vitro. The laser stimulator offered the
unique opportunity to test whether the same stimuli also elicit pain sensations
in humans.A specific heatinduced current (Iheat) was elicited in six of 13 small
DRGneurons (diameter#30 mm) tested in the whole-cell configuration of the patch–clamp
mode. Current responses in the seven heat-insensitive neurons were within the
range explainable by the temperature dependence of the recording setup. Iheat
was characterized by: (1) non-linearity of its amplitude during a suprathreshold
heat ramp as well as with stimuli of increasing intensity with an estimated
threshold of 42 ^ 18C; (2) fast rise time and even faster decay time (t1=2 ¼
96:5 ^ 5:9 and 27.7 ^ 1.5 ms, respectively); and (3) rate dependence of its
induction. All three heat-sensitive neurons tested were also sensitive to
capsaicin. The mean threshold for the induction of Iheat was 2.8 ^ 0.3 J mm22.
The threshold for the induction of action potentials by depolarizing current
pulses was significantly reduced after laser stimulation, suggesting a
sensitization at the transformation stage. No such change was seen in
heat-insensitive neurons that underwent the same heat stimuli. The same diode
laser elicited pain sensations and laser-evoked potentials in human subjects. No
significant differences were seen between the pain thresholds in hairy and in
glabrous skin, probably due to the deep penetration of this laser radiation. The
mean pain threshold for stimuli $200 ms in humans was 2.5 ^ 0.2 J mm22 (n ¼
11), and did not differ from the thresholds for the induction of Iheat in vitro.
Our results indicate that Iheat in primary sensory neurons can be activated by
infrared laser pulses that are painful in humans. q 2002 Published by Elsevier
Science B.V. on behalf of International Association for the Study of Pain.
Infrared
diode laser pulses that are painful for human subjects, activate an inward
current in primary nociceptive neurons in vitro that shares many properties with
Iheat that has been studied with application of heated solutions. As Iheat is
likely mediated by opening of the vanilloid receptor channel VR1, brief heat
stimuli generated by infrared lasers offer the opportunity to study the
functional integrity of VR1-expressing nerve fibers in humans. The LEP data in
humans showed a latency that corresponded to activation of myelinated afferents.
This observation suggests that the diode laser pulses activated type II A-fiber
mechano-heat nociceptors, a class of polymodal nociceptive afferents that are
thought to express the fast Iheat and VR1. Diode lasers are adaequate as
non-contact heat stimulators for human pain research, which rapidly activate
capsaicin-sensitive nociceptive afferents relatively independent of their depth
of termination within the skin.

